The crystalline nanoparticles of Zn0,8Ni0,2Fe2O4 ferrite were first synthesized by chemical co-precipitation with concentration 0,1M of precursors, then modified by 0.5M solution of oleic acid in pentanol and finally heated at temperatures of 80, 200, 300 and 400 oC for 6 h…
NGUYEN VAN DAN*, HOANG THI QUYNH TRANG AND LUONG THI QUYNH ANH
Department of Metallic Materials, Faculty of Materials Technology, HCMUT-VNUHCM, 268 Ly Thuong Kiet, Ward 14, District 10, Ho Chi Minh City, Vietnam
*Email: ngvdan@hcmut.edu.vn
Ngày nhận bài: 6/9/2017, Ngày duyệt đăng: 21/11/2017
ABSTRACT
The crystalline nanoparticles of Zn0,8Ni0,2Fe2O4 ferrite were first synthesized by chemical co-precipitation with concentration 0,1M of precursors, then modified by 0.5M solution of oleic acid in pentanol and finally heated at temperatures of 80, 200, 300 and 400 oC for 6 h. The effect of heating temperature on crystallite size and magnetic properties of ferrite was studied. The analysis results of X- Ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) of the samples showed that only the samples heated at 200, 300 and 400 oC are crystallized and crystallite size is changed with corresponding values of 6, 9 and 11 nm. The magnetic properties of Zn0,8Ni0,2Fe2O4 ferrite measured by vibrating sample magnetometer (VSM) showed that for samples heated at 200 and 300 oC, the coercive force Hc and remanence M are about zero while the sat- uration magnetization Ms has values of 25 and 47.18 emu/g, respectively.
Keywords: crystalline nanoparticles, Zn0,8Ni0,2Fe2O4 ferrite, superparamagnetism, magnetic properties
INTRODUCTION
The superparamagnetic nanoparticles of Zn-Ni- Fe ferrite system are used currently for special applications such as target-directed medicine, cancer treatment, increased resolution of magnet- ic resonance imaging, improved radar wave absorption, ferrofluid, etc. [1,2]. Superparamagnetism (SPM) is a type of magnet- ism that occurs in small ferromagnetic or ferrimag- netic nanoparticles. Crystalline size of SPM is around a few nanometers to 10 nm, depending on the material. In the superparamagnetic nature, fer- romagnetic or ferrimagnetic nanoparticles have coercive force Hc = 0 and remanence Mr = 0 like paramagnertic materials, but their saturation mag- netization is many times higher than that of the paramagnertic materials [3, 4].
There are some of methods used for the syn- thesis of ferrite nanoparticles such as: co-precipi- tation, sol-gel method, hydrothermal technique, etc. [5-7].
In this paper, nanoparticles of Zn0.8Ni0.2Fe2O4 ferrite were first synthesized by chemical co-precipitation, then modified by oleic acid and finally heated at different temperatures. This study aims to synthesize the superparamagnetic nanoparticles and to understand how the heating temperature influences crystallite size and magnetic properties of the ferrite.
EXPERIMENTAL
2.1. Chemicals
The following chemicals such as FeCl3.6H2O – Merck (Germany), ZnCl2 – Merck (Germany), NiCl2.6H2O – Merck (Germany), 1 – Pentanol (C5H12O) – Merck (Germany), n-Hexane (C6H14) – (China), Oleic acid (C18H34O2) – Sigma aldrich (US) and Ethanol – (China) were used for synthesis.
2.2. Synthesis of Zn0.8Ni0.2Fe2O4 ferrite
Each salt type was dissolved in double distilled water to the concentration 0.1M. Solution of each salt was mixed and heated at 60 oC with a stirring speed of 500 rev/min. A 25 % ammonia solution was then added to the above mixture drop by drop while stirring kept constant. The addition of ammonia will stop when pH maintained at 8.5. To avoid agglomeration of particles, 0.5 M solution of oleic acid in pentanol was dropped in the mixture at 60 oC with stirring. The mixture was then heated to 80 oC and stirring was stopped when the smell of ammonia disappears (about 1 h) Products were obtained in the form of precipitates and then separated from water and washed several times by re- dispersing in n-hexane and precipitating with ethanol to remove salt residues and other impurities. Finally to study the effect of heating temperature on crystallite size and magnetic properties of ferrite, the powder was heated at temperatures 80, 200, 300 and 400 oC for 6 h. The formation of ferrite nanoparticles passed a two- step process:
In the first step, hydroxide nanoparticles were co-precipitated from the metal salts and ammonia solution, and then were capped (overlayed) from each other by oleic acid (co-precipitation step). The oleic acid initially reacted with the ammonia to form ammonium oleate. Additional heating decomposed ammonium oleate to ammonia gas and oleate ions, which attached to and surround the hydroxide nanoparticles. The mentions above can be expressed in the following chemical reactions:
0,2Ni2+ + 0,8Zn2+ + 2Fe3+ + 8OH¯ → 0,2Ni(OH)20,8Zn(OH)22Fe(OH)3↓ (1)
NH4OH + C17H33COOH → C17H33COONH4 +H2O (2)
C17H33COONH4 heating→ C17H33COOH + NH3 (3)
C17H33COO+Ni2+/Zn2+/Fe3+ heating→ C17H33COO (Ni/Zn/Fe) (4)
The second step consists of transformation of hydroxides into nanoferrites occurring when the samples were heated at appropriate temperatures (ferritization step):
0,2Ni(OH)2.0,8Zn(OH)2.2Fe(OH) heating→ Ni0.2Zn0.8Fe2O4 + 4H2O (5)
At this step, the crystalline nanoparticles of fer- rite are formed and grew up. The growth of crys- talline nanoparticles of ferrite is hindered by covered layer of metal oleate. As a result, crystalline nanoparticles of ferrite are obtained at appropriate heating temperature.
2.3. Characterization
Structure of ferrite was studied by X-ray diffraction (XRD). Microstructure was observed by scan- ning electron microscope (SEM) and transmission electron microscope (TEM). Chemical composi- tion of ferrite was determined by energy-dis- persive X-ray spectroscopy (EDS). Magnetic prop- erties of ferrite were measured by vibrating sample magnetometer (VSM).
sample heated at 400 oC
RESULTS AND DISCUSSION
3.1. Chemical composition
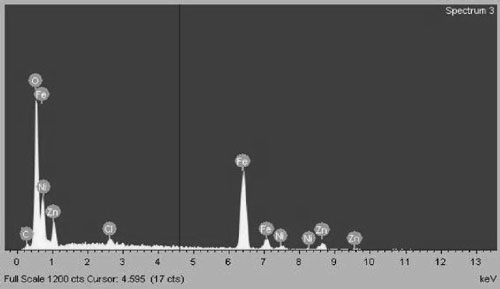
Chemical composition of Zn0.8Ni0.2Fe2O4 sam- ples was determined by EDS. Figure 1 shows EDS spectra of a sample heated at 400 oC which con- firms appearing of Fe, Zn, Ni and O.
The table 1 shows chemical composition of Zn0.8Ni0.2Fe2O4 sample heated at 400 oC and determined by EDS compared to theoretical composition. The table shows also no difference between them, but traces of salt and oleic acid residues were found with negligible levels.
Table 1. Chemical composition of Zn0.8Ni0.2Fe2O4 sample heated at 400 oC
| Element | Weight by EDS (%), | Theoretical weight (%) |
| O K | 26.50 | 26.71 |
| Fe K | 47.84 | 46.74 |
| Ni K | 4.84 | 4.84 |
| Zn K | 21.70 | 21.70 |
3.2. Crystallite structure and its size
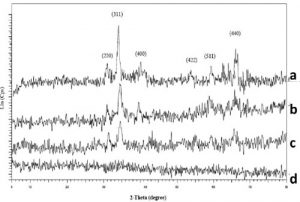
Structure of samples was studied by X-ray dif- fraction (XRD). Figure 2 shows diffraction pat- terns for as-synthesized samples heated at dif- ferent temperatures: 80, 200, 300 and 400 oC for 6 h. Observed diffraction peaks of the samples heated at 200, 300 and 400 oC are correspond- ing to the (220), (311), (400), (422), (511) and (440) standard powder diffraction lines of Fe-Zn- Ni ferrite [8]. The sample heated at 80 oC is not crystallized because all of the diffraction peaks are not observed.
The crystallite size of Zn0.8Ni0.2Fe2O4 ferrite was determined by Scherrer formula [9]:
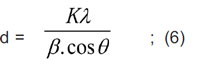
where: d is the average crystallite size; K = 0.89; λ is the wavelength of X-ray, λCu (Kα) = 1.54056 Å; β is the full width at half of the diffraction peak with maximal intensity (FWHM) in radians; θ is the Bragg angle of diffraction at the peak
with maximal intensity (2θ = 35.64o).
Values of the average crystallite size corresponding to β of samples heated at 200, 300 and 400 oC are showed in table 2.
Table 2. The average crystallite size of samples heated at 200, 300 and 400 oC determined by Scherrer formula
| Samples | β (FWHM) (radian) | Crystallite size (nm) |
| Heated at 200 oC | 1.403 | 6 |
| Heated at 300 oC | 1.012 | 9 |
| Heated at 400 oC | 0.676 | 11 |
3.3. Microstructure
Microstructure of Zn0.8Ni0.2Fe2O4 samples heated at different temperatures: 200, 300 and 400 oC for 6 h was studied by TEM. Prior to the TEM analysis the powders were dispersed in n- hexane and then treated by ultrasonication for about 10 min. A few drops of the suspension were placed on a carbon-coated copper grid and left to dry in the air. Figure 3 shows the TEM images of samples heated at different temperatures: 200, 300, 400 oC for 6 h

The average crystallite sizes of Zn0.8Ni0.2Fe2O4 ferrite nanoparticles heated at 400, 300 and 200 oC can be also analyzed by software TEM image J and shown in table 3.
Table 3. The average crystallite size of samples heated at 200; 300 and 400 oC analyzed by soft- ware TEM image J
| Samples | Average crystallite size (nm) |
| Heated at 200 oC | 6.5 |
| Heated at 300 oC | 8 |
| Heated at 400 oC | 11 |
Comparing values of the average crystallite sizes of samples heated at 200, 300 and 400 oC determined by Scherrer formula and analyzed by software TEM image J in table 2 and table 3 shows very good agreement.
These tables also demonstrate that increase of heating temperature from 200 to 400 oC leads to incresing the average crystallite size of ferrite from 6 to 11 nm. This is explained by the growth of crys- tallite nanoparticles of ferrite with increasing heating temperature.
3.4. Magnetic properties
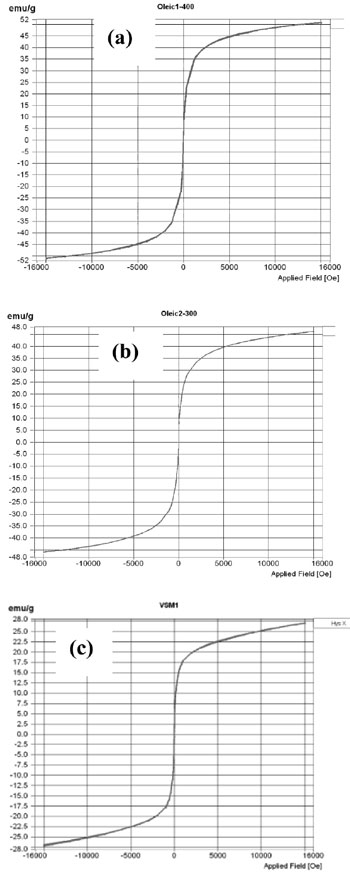
Magnetic properties of Zn0.8Ni0.2Fe2O4 ferrite nanoparticles were measured by vibrating sample magnetometer (VSM). Figures 4 shows magneti- zation curve of nanoferrite samples crystallized at 200, 300 and 400 oC.
Magnetic properties of Zn0.8Ni0.2Fe2O4 ferrite nanoparticles are coercive force (Hc), remanence (Mr) and saturation magnetization (Ms) given in table 4.
Table 4. Magnetic properties and crystallite size of Zn0.8Ni0.2Fe2O4 ferrite samples crystallized at 200, 300 and 400 oC
| Samples | Mr (emu/g) | Ms (emu/g) | Hc (Oe) | d (nm) |
| Crystallized at 200 oC | 0 | 27.11 | 0 | 6.5 |
| Crystallized at 300 oC | 0 | 47.18 | 0 | 8 |
| Crystallized at 400 oC | 0.027 | 50.82 | 4.31 | 11 |
Table 4 shows that the Zn0.8Ni0.2Fe2O4 ferrite nanoparticles crystallized at 200 and 300 oC having zero coercive force Hc and remanence Mr are of superparamagnetic nature. The superparamag- netic nature of ferrite can be attributed by their small crystallite size [3, 4]. As mentioned above, crystallite size of SPM is around a few nanometers to 10. With such a small crystallite size, the crystalline nanoparticles have greater thermal energy compared to magnetic anisotropic energy and the magnetic moment of the nanoparticles fluctuates like the paramagnetic materials [3, 4]. The Zn0.8Ni0.2Fe2O4 ferrite nanoparticles crystallized at 400 oC are not superparamagnetic because their crystallite size was increased up to 11 nm.
In addition, tables 2, 3 and 4 also show that when the crystallization temperature dropped from 400 down to 200 oC, the crystallite size decreased from 11 to 6.5 nm and corresponding saturation magnetization decreased from 50.82 to 27.11 emu/g. The more heating temperature is reduced, the smaller is crystallite size of ferrite. This is explained by the growth of crystalline nanoparticles of ferrite as mentioned above. The more crystallite size of ferrite is decreased, the smaller saturation magnetization is observed, because the magnetic energy is proportional to the crystal volume.
The magnetic properties of Zn-Ni ferrite nanoparticles are different from that of bulk ferrite. The magnetic saturation (Ms) of the Zn-nanoferrite was lower than that of the bulk one [11]. However, the coercivity (Hc) and remanance (Mr) of Zn-Ni ferrite nanoparticles with crystallite size lower than 10 nm reached zero value suitable for the super- paramagnetic state, while these values of bulk Zn- Ni ferrite are too high [10, 11] for superparamag- netism.
4. CONCLUSIONS
The superparamagnetic nanoparticles of Zn0.8Ni0.2Fe2O4 ferrite with crystallite size of about (6 9) nm, zero coercive force Hc and remanence Mr and saturation magnetization Ms from 27.11 to 47.18 emu/g have been successfully synthesized corresponding to finally heating temperatures at 200 and 300 oC for 6 h.
Increasing the heating temperature to 400 oC or higher leads to increase of the crystallite size of ferrite over 10 nm that makes disappear the super- paramagnetic properties of ferrite. Thus, only the ferrite with nanoparticle size below 10 nm demon- strated the superparamagnetic behavior with zero Hc and Mr.
Acknowledgement. This research is funded by the Ho Chi Minh City University of Technology – VNU-HCM under grant number T-CNVL-2016- 103.
REFERENCES
- Alex Goldman, Modern ferrite technology, Springer Science, Business Media Inc, 2006, p.438
- Lu A. H., Salabas E. L., Schuth F., Magnetic nanoparticles: synthesis, protection, functionalization and appli- cation, Angewandte Chemie-International Edition, 46 (8), 2007, pp.1222-1244
- Manuel Benz, Superparamagnetism: theory and applications, Research gate, 2012
- Meizhen Gao, Wen Li, Jingwei Dong, Zhirong Zhang, Bingjun Yang, Synthesis and characterization of super- paramagnetic Fe3O4@SiO2 core-Shell composite nanoparticles, World Journal of Condensed Matter Physics, 1, 2011, pp. 49-54
- K. Bhattacharjee K. and Ghosh C. K. – Novel synthesis of NixZn1-xFe2O4 (0 < x < 1) nanoparticles and their dielectric properties, J Nanopart Res, 13, 2011, pp.739-750
- Sanjay Kumar, Simple synthesis and magnetic properties of nickel-zinc ferrites nanoparticles by using Aloe vera extract solution, Archives of Applied Science Research, 5 (6), 2013, pp.145-151
- Sonja Jovanovich, Matjaž Spreitzer, Melita Tramsek, Zvonko Trontelj and Danilo Suvorov, The effect of oleic acid concentration on the physicochemical properties of cobalt ferrite nanoparticles, J. Phys. Chem. C, 118 (25), 2014, pp.13844-13856
- David Jiles, Introduction to magnetism and magnetic materials, Published by Chapman & Hall, 1994, pp.430
- Cullity B. D; Element of X-ray diffraction, Adison – Wesley, 1956, pp. 531
- Chandan Upadhyay and H. C. Verma; Cation distribution in nanosized Ni-Zn ferrites, Journal of applied physics, volume 95, number 10, 15 May 2004
- H. E. Zhang, B. F. Zhang, G. F. Wang, X.H. Dong and Y. Gao; The structure and magnetic properties of Zn1-xNixFe2O4 ferrite nanoparticles prepared by sol-gel auto-combustion, Journal of magnetism and magnetic materials, 312, 2007, pp.126-130