Trong nghiên cứu này, ảnh hưởng của nhiệt độ tới tuổi thọ của dung dịch polyme PVP được đánh giá dựa trên phân tích đường nguội, độ nhớt động lực học, khối lượng phân tử.
Effect of thermal degradation on the lifetime of the aqueous polyvinyl pyrrolidone quenchant
TRẦN THỊ XUÂN1, NGUYỄN VĂN TƯ1, VŨ ĐÌNH TOẠI1,*
1Viện Khoa học và Kỹ thuật Vật liệu, Trường Đại học Bách khoa Hà Nội, Số 1 Đại Cồ Việt, Hà Nội
*Email: toại.vudinh@hust.edu.vn
Ngày nhận bài: 21/2/2019, Ngày duyệt đăng: 18/3/2019
ABSTRACT
Polymer quenchants are becoming more and more popular as substitutes for traditional quenching media in heat treatment. Especially, water-soluble organic polymer offers several environmental, economic, and technical advantages, as well as eliminates the quench-oil fire hazard. Furthermore, the cooling characteristics of polymer quenchant are influenced by concentration, agitation, temperature, degradation, contamination and drag-out. In the present study, the influence of temperature on the ageing of aqueous polyvinyl pyrrolidone (PVP) was evaluated regarding cooling curves, kinematic viscosity, molecular weight. The cooling curves were determined by the ISO 9950:1995 standard, the molecular weight of PVP was calculated based on nuclear magnetic resonance (NMR) spectroscopy. The results showed that after 30, 60, 150 and 200 quenching cycles, the viscosity of the polymer PVP solution decreases by about 2, 5, 17 and 30 %, respectively. The observed NMR spectrums indicated that the polymer chains were shorter and the molecular weight of the PVP was decreased after many cycles of quenching.
Keywords: Polyvinyl pyrrolidone, nuclear magnetic resonance (NMR), quenchant.
TÓM TẮT
Dung dịch tôi polyme đang ngày càng được sử dụng phổ biến và dần thay thế cho môi trường tôi truyền thông trong công nghệ xử lý nhiệt. Đặc biệt, dung dịch polyme hữu cơ tan trong nước thân thiện với môi trường, tính kinh tế cao, có nhiều ưu điểm công nghệ và không bắt cháy như dầu. Hơn nữa, tốc độ nguội của dung dịch tôi polyme còn bị ảnh hưởng bởi nồng độ, tốc độ khuấy, nhiệt độ và sự thoái hóa của polyme. Trong nghiên cứu này, ảnh hưởng của nhiệt độ tới tuổi thọ của dung dịch polyme PVP được đánh giá dựa trên phân tích đường nguội, độ nhớt động lực học, khối lượng phân tử. Đường nguội của dung dịch PVP trong nước được xác định theo tiêu chuẩn ISO 9950:1995 và khối lượng phân tử của PVP được tính toán dựa vào phổ cộng hưởng từ hạt nhân (NMR). Kết quả cho thấy, sau 30, 60, 150 và 200 lần tôi, độ nhớt của dung dịch polyme PVP trong nước giảm tương ứng là 2, 5, 17 và 30 %. Kết quả từ trên phổ NMR cho thấy chuỗi polyme PVP ngắn hơn và khối lượng phân tử giảm sau nhiều lần tôi.
Từ khóa: Polyvinyl pyrolidone, cộng hưởng từ hạt nhân (NMR), môi trường tôi.
1. INTRODUCTION
The use of aqueous polymer quenchants is increasing in heat-treating industries for quenching of aluminum alloys and steels, as substitutes for both water and oil quenchants. The polymer quenchants are prepared by dissolving the organic polymer in water. Polymer quenchants have the advantages of easy handling, better stability, and consistent cooling performance over a period of time [1]. The cooling characteristics of polymer quenchants are intermediate between water and oil, their Grossmann quench severity is in the range of 0.2-1.2 [2] The use of commercial PVP polymer as quenchant begun in 1975 [3]. PVP is non-toxic, water soluble, biologically compatible and hence it is eco-friendly [4]. PVP has similar performance compared to mineral oil, with good stability in use; its solubility in water is high, with higher drag-out than polyalkylene glycol (PAG) at the same viscosity [5].
The cooling characteristics of polymer quenchant are influenced by concentration, agitation, temperature, thermal degradation, contamination, and drag-out. Many researchers such as Z. Koudil et al., Rafika Ikkene et al. have investigated the effect of PVP concentrations, bath temperatures, agitation speeds on the cooling rate of the solution [3]. These studies showed that when the concentration of PVP decreased, the cooling rate of the solution increased. On the other hand, as the temperature of the quenching bath and the agitation speed increased, the cooling rate also increased. E. Troell et al. had reported the effect of thermal oxidation of the PAG quenchant on its cooling rate and viscosity by measuring the viscosity and cooling rate of PAG polymer quenchant after the number of quenching times [6]. The results revealed that after more times of quenching, the viscosity of the solution decreased while the cooling rate increased. George E. Totten, by using the Fourier Transform Infra-Red (FTIR) method, also noted that after some quenching cycles, the PAG polymer in the quenchant was oxidized [7]. In summary, the previous studies have shown that, due to the effect of the temperature, the chain of the polymer was heat degraded, leading to reduce the life-time of the quenchant. However, there has been no quantitative study of the change in polymer molecular weights in the quenchant under the influence of the temperature.
In this paper, the effect of thermal degradation on the lifetime of the aqueous polyvinyl pyrrolidone quenchant was investigated by evaluating the changing of cooling rate, viscosity and polymer molecular weight of the 4 % PVP quenchant after 30, 60, 150 and 200 quenching cycles. The changing of the polymer molecular weight as well as the lifetime of PVP quenchant was determined based on the measured results about the cooling rate, the viscosity and the Nuclear Magnetic Resonance spectroscopy (NMR).
2. EXPERIMENTAL
In this study, the aqueous solutions which dissolved the PVP K60 polymer with a concentration of 4 wt.% was used as the polymer quenchant. The average weight of PVP K60 polymer is Mw = 280000 (g/mol) and the chemical structure of PVP polymer is shown in Fig.1 [4].
To evaluate the lifetime of the researched PVP polymer quenchant (4 %), it is necessary to measure the cooling rate of the initial solution and the solutions after 30, 60, 150 and 200 quenching cycles. The cooling rate of the PVP polymer quenchant was determined following the experimental diagram in Fig.2. The experimental system consists of a furnace equipped with a temperature controller, one standard probe was made by the Inconel 600 alloy and fabricated in accordance with ISO 9950 standard (equivalent to ASTM D6200) about structure, shape, and dimensions; two thermocouple wires were mounted in the core of probe and connected to the temperature recording device; one temperature versus time recording device (USB 4718) was connected to the computer and WaveScan software. The measurement diagram is shown in Fig. 2.
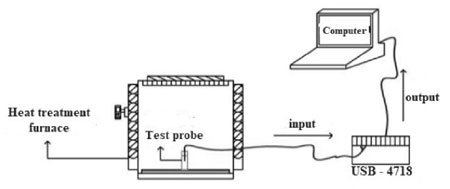
When the standard probe (Inconel 600) is heated to 850 oC, it is lifted off the furnace and immediately immersed into the 4 % PVP polymer quenchant, while the temperature recording device records the temperature of the thermocouple wires (mounted in the core of probe) continuously with a time step of 0.5 second; at the same time these signals are sent to the computer and the WaveScan software will draw a cooling curve describing the cooling process of the standard probe in the quenchant. Based on this cooling curve, the cooling rate of the quenchant can be calculated. Besides, the temperature of the quenchant will change, simultaneously there is an amount of water will evaporate from the solution in the quenching process. On the other hand, because the cooling rate of the quenchant depends on the temperature and the volume of the solution, the temperature of the quenchant at the starting measuring is always kept at 30 oC and the volume of solution is always added to stabilize at 1.5 liters in the measurement process to achieve high accuracy. The volume of quenchant has been calculated so that in quenching process, the probe (with the standard size and the temperature of 850 °C) is sufficiently cooled in the quenchant but the temperature of the quenchant does not exceed 60 °C. The samples of PVP polymer quenchant (4 %) with no quenching cycles, and after 30, 60, 150 and 200 quenching cycles were taken to equal volumes (100 ml) to analyze the viscosity, and nuclear magnetic resonance spectrometry (NMR). In this research, the viscosity of the quenchant was measured by using the Brookfield DVE device (USA). The molecular weight of the PVP is determined by the 1H-NMR spectra using the Jeol ECA 400 MHz spectrometer (Japan).
3. RESULTS AND DISCUSSION
3.1. Cooling rate of the 4% PVP quenchant after several numbers of quenching cycles
The cooling rate of the 4% PVP quenchant after several numbers of quenching cycles is presented in Fig. 3. The results show that when the quenchant has been quenched many times, the maximum cooling rate (CRmax) increased and the cooling rate at 300 °C also increased.
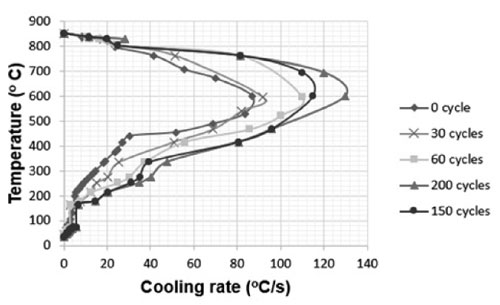
Some important cooling parameters of the 4 % PVP K60 quenchant extracted from the cooling rate curves after several numbers of quenching cycles are shown in Table 1, where CRmax is the largest cooling rate, T is the temperature at which the maximum cooling rate is achieved and CR300 is the cooling rate at 300 °C. The results in Table 1 indicated that the temperature with the maximum cooling rate of the 4 % PVP quenchant after 30, 60, 150 quenching cycles was not greater than the initial solution (about 600 °C). However, this temperature increased to 630 °C after 200 quenching cycles. In this case, the maximum cooling rate of the quenchant was 130 °C/s and increased about 49 % in comparison with the initial solution. This problem can be explained by the fact that after a number of quenching cycles the polymer in the solution is affected by the temperature, especially the polymer layer near the quenched detail was oxidized and the chain (chemical bond) was bro- ken that reduced the molecular weight, led to the reduction of the viscosity and increased the cooling rate of the PVP quenchant.
Table 1. Cooling parameters of the 4% PVP K60 quenchant extracted from the cooling rate curves after several quenching cycles
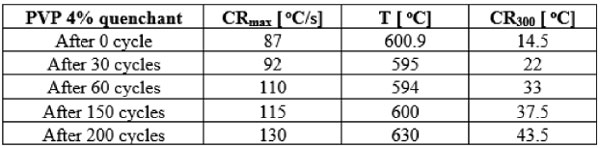
3.2. The viscosity of the initial solution and the solution after several numbers of quenching cycles
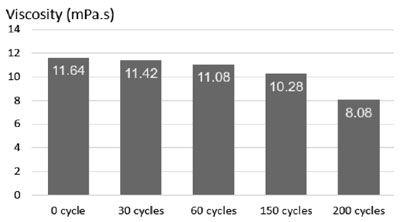
Fig. 4 exhibits the results of the viscosity measurements of the initial solution and the solution after 30, 60, 150 and 200 quenching cycles. The results showed that when the solution was quenched with more cycles, the lower viscosity was achieved i.e. after 30, 60, 200 quenching cycles, the viscosity was decreased by 2 % (from 11.64 to 11.42 mPa.s), 5 % (from 11.64 to 11.08 mPa.s) and 31 % (from 11.64 to 8.08 mPa.s), respectively. The reason of the viscosity reduction was due to the decreasing of polymer molecular weight in the quenchant and the small amount of polymer attached to the quenched detail was removed from the solution. It is clear seen that the viscosity of the quenchant affected its cooling rate directly.
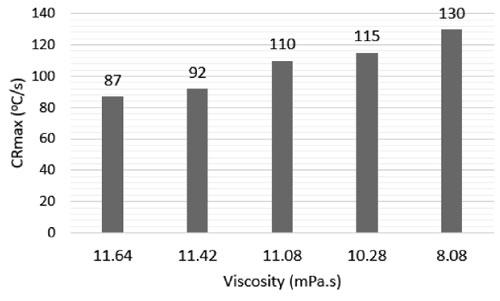
Fig. 5 presents the relationship between the viscosity and the maximum cooling rate (CRmax) of the PVP quenchant. In the case of PVP quenchant, the lower the viscosity is, the faster the cooling speed is.
3.3. Molecular weight of PVP after 0, 150, 200 quenching cycles
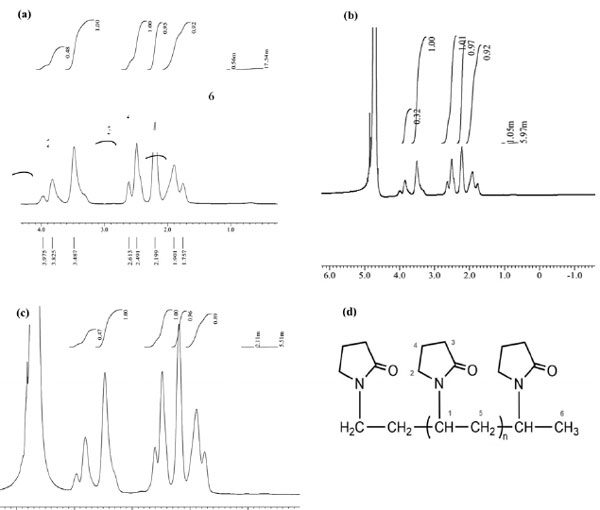
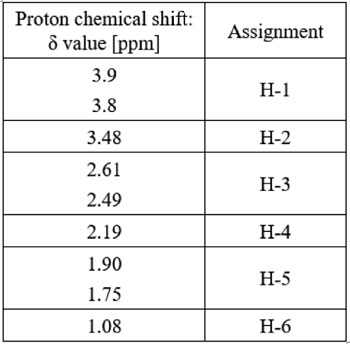
The 1H-NMR spectrum gave the information about the types of protons, the number of each type of proton and their chemical environment, whereby it assisted to reach the important conclu- sions about the structure of the polymer. The 1H- NMR spectrum of PVP quenchant at 0 (a), 150 (b), 200 (c) quenching cycles and the proton type in PVP (d) is displayed in Fig. 6. The proton chemical shift of PVP is presented in Table 2.
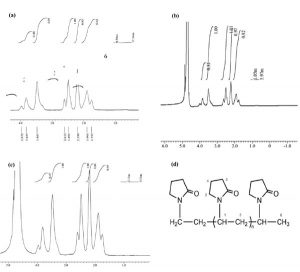
Table 2. Proton chemical shift of PVP
According to the reference [8], the molecular weight of polymer could be determined by following formula:
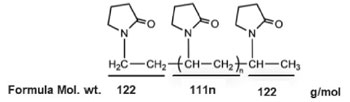
Mw = 112×2 + 111×n (1)
where n is the number of repeating units of PVP block which could be calculated as below:
![]() (2)
(2)
QUOTE n = proton H1 intensity (2) Through equation (2) we obtained the value of n. Besides, based on equation (1) and equation (2), and the 1H-NMR spectrum of the PVP poly- mer after the number of quenching cycles Fig. 6, the molecular weight of PVP polymer was calcu- lated as shown in Table 3.
Table 3. Molecular weight of PVP after quenching cycles
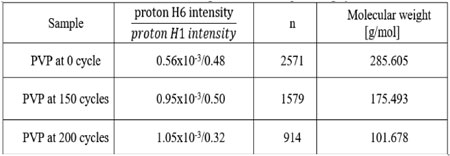
From the calculated results in Table 3, it can be seen that the molecular weight of PVP in the aque- ous polymer quenchant decreased gradually over a number of the quenching cycles. The molecular weight of the PVP initial quenchant was obtained about 285.605 (g/mol) with the molecular weight in the range of PVP K60. After 150 and 200 quenching cycles, the molecular weight of PVP decreased by 38 % to 175.493 (g/mol) and 64 % to 101.678 (g/mol), respectively. The results suggested that with the effect of the temperature in quenching process, the polymer chains broken and shortened, simultaneously the molecular weight of PVP reduced gradually as the solution underwent the bigger number of quenching cycles. This was also the main reason why the viscosity of the quenchant decreased and the cooling rate of the quenching medium increased after many cycles of quenching.
4. CONCLUSION
The cooling curve is the standard for evaluating the lifetime of the quenchant. The cooling rate of the PVP polymer quenchant gradually enhanced, which was meant that the lifetime of the PVP polymer quenchant regularly decreased. Due to the effect of temperature, the polymer molecules were broken and damaged, which in turn causing the polymer chain shortened and the molecular weight decreased. In other words, the polymer quenchant has been undergone to the thermal degradation. To ensure that the cooling rate was suitable with the requirements of the quenched detail, adding an amount of polymer to the quenchant after a certain number of quenching times was needed. Based on this research, it is possible to determine when and how much polymers need to be added to the quenchant.
REFERENCES
- Rafika Ikkene, Zahia Koudil, and Mohammed Mouzali; Cooling characteristic of polymeric quenchant: Calculation of HTC and prediction of microstructure and hardness, JMEPEG, No.23, 2014, p. 3819–3830.
- G. E. Totten, C. E Bates, and N. A. Clinton; Measuring hardenability and quench severity, in: Chap. 2, Handbook of Quenchants and Quenching Technology, ASM International, Materials Park, OH, 1993.
- Z. Koudil, R. Ikkene, and M. Mouzali, Cooling Capacity Optimization: Calculation of Hardening Power of Aqueous Solution Based on Poly(N-Vinyl-2-Pyrrolidone), JMEPEG, Vol. 23, 2014, p. 551–559.
- K. V. Anasuya, M. K. Veeraiah, P. Hemalatha, M. Manju; Synthesis and characterisation of poly (Vinylpyrrolidone) – Nickel (II) complexes, IOSR Journal of Applied Chemistry, Vol. 7, No. 8, 2014, p. 61-66.
- Xuan Thi Tran, Toai Dinh Vu, Khanh Quoc Dang; Numerical simulation of the heat treatment process for 100Cr6 steel, Acta Metallurgica Slovaca, Vol. 23, No. 3, 2017, p. 236-243.
- E. Troell, H. Kristoffersen; Influence on cooling characteristics of ageing and contamination of polymer quen- chant, Berg- und Huttenmannische Monatshefte (BHM), Vol. 155, No. 3, 2010, p. 114-118.
- V. Rudnev, G. E. Totten; Polymer quenchants for induction heat treating applications: The basic, induction heat- ing and heat treatment, ASM Handbook, Volume 4C, 2014.
- Josephat U. Izunobi and Clement L. Higginbotham; Polymer molecular weight analysis by 1H NMR spec- troscopy, Journal of Chemical Education, Vol. 88, No. 8, 2011, p. 1098-1104.